UC Davis Bioanalysis and Pharmacokinetics Core Facility
Department of Neurology
University of California, Davis School of Medicine
|
The UC Davis Bioanalysis and Pharmacokinetics Core Facility provides bioanalysis of diverse analytes in blood and other human and animal tissues. The facility serves the needs of the UC Davis Medical Center in Sacramento and also external entities, including universities, research institutes, and commercial organizations.
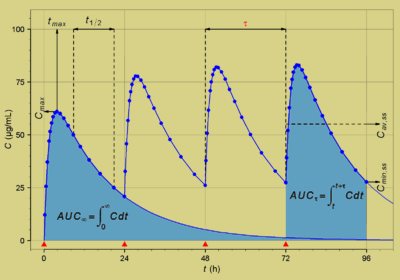
The facility contains a Waters Acquity-I class UPLC and Waters Xevo TQ-S triple quadrupole MS/MS with ESI and APCI/APPI ionization sources. Pharmacokinetic analyses are performed with Centara Phoenix WinNonlin 7.0, an industry-standard and FDA-compliant software system for pharmacokinetics. The facility conducts analytical services for the UC Davis Good Manufacturing Practices Laboratory, allowing the production of drug products meeting FDA, USP and State of California Requirements. |
Facility Director: Chun-Yi "Jimmy" Wu, Ph.D, project scientist, Department of Neurology, University of California, Davis School of Medicine
Faculty Sponsor: Michael A. Rogawski, M.D., Ph.D., professor of neurology and pharmacology, University of California, Davis School of Medicine |